
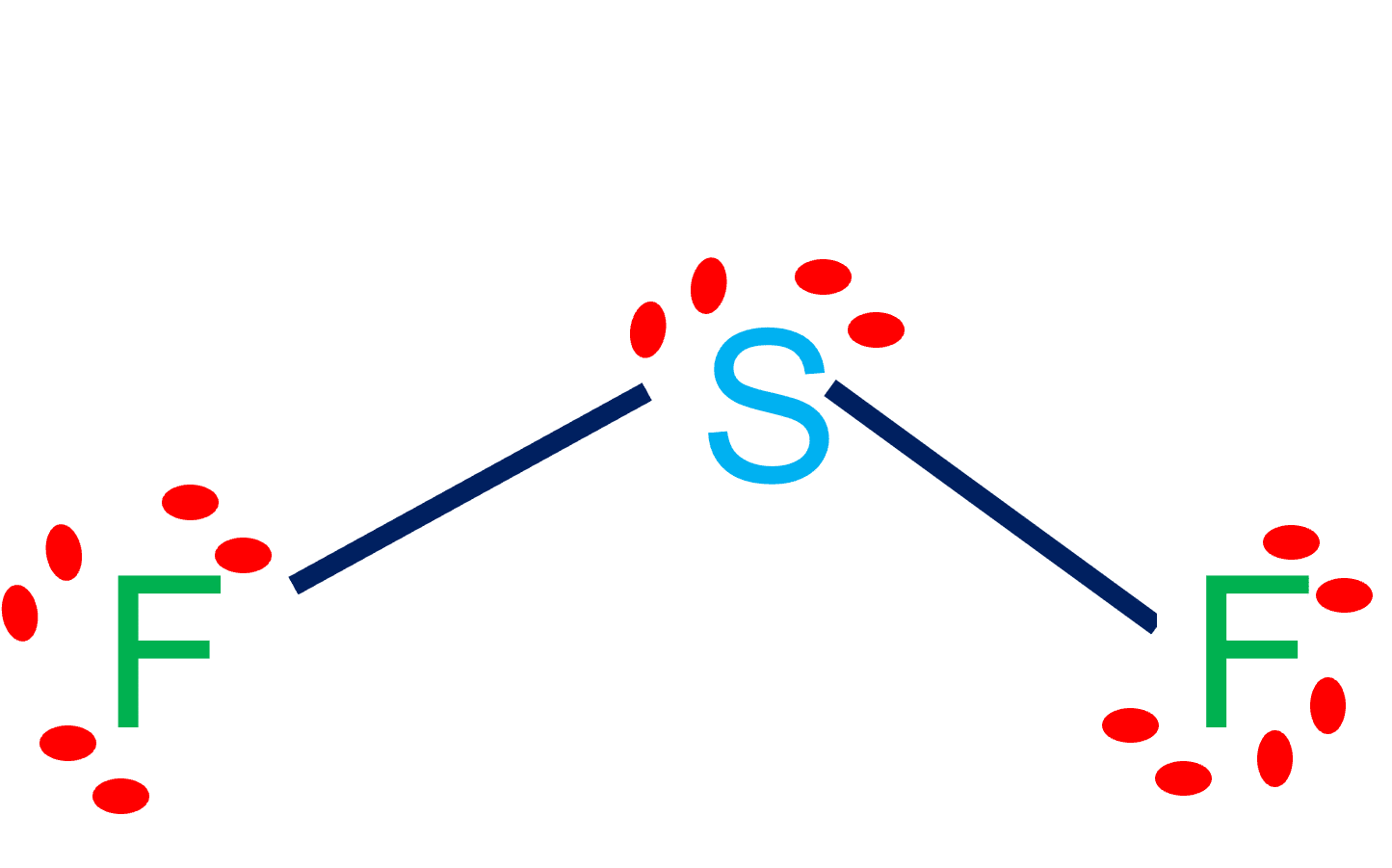
SOLVED62. The molecule SF2 is bent Is it polar or nonpolar? Explain
Here's how you can easily draw the SF 2 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let's take a closer look at each step mentioned above.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
(Xenon difluoride) - YouTube 0:00 / 2:33 Is XeF2 Polar or Nonpolar? (Xenon difluoride) Wayne Breslyn 723K subscribers Join Subscribe Subscribed 16K views 2 years ago Learn to determine if XeF2.

Sf2 polar or nonpolar limfamike
Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Geometry of Molecules 2.05K subscribers Subscribe 6 Share 1K views 1 year ago Polarity of Molecules Hey Guys! In this video, we are going to.

SF2 Lewis structure, Molecular geometry, Hybridization, Polar or nonpolar
SF2) molecule is classified as a polar molecule. The molecule of sulfur difluoride (with tetrahedral or bent V-shaped molecular geometry) is tilted, the bond angles between sulfur and. Molecules can be classified as polar or nonpolar. The molecule polar behaves in a different manner as compared to nonpolar. Overview:

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
Step 4: Finally, we need to see if these dipole moments cancel out. In a linear molecule, the dipole moments can cancel out, making the molecule nonpolar. However, in a bent molecule like $\mathrm{SF}_{2}$, the dipole moments do not cancel out. Therefore, the molecule $\mathrm{SF}_{2}$ is \textbf{polar}.

Sf2 molecule polar or nonpolar? [Expert Review]
S2F2 is polar or non polar? Updated: 8/11/2023 Wiki User ∙ 10y ago Study now See answers (4) Best Answer Copy Think of the sulfite ion as a molecule with its geometry and dipole moment AND a.

Sf2 polar or nonpolar limfath
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.
Sf2 polar or nonpolar lenaka
Molecular geometry or shape How do oppositely charged poles develop in an SF2 molecule, and how do the three factors given above contribute to its polar nature? Let's find out in a detailed discussion. Factors affecting the polarity of SF2 Electronegativity

So far, we’ve used 20 of the SF2 Lewis structure’s total 20 outermost
January 17, 2022 by NCERT Point Team Answer In order to evaluate whether or not SF2 is polar, we must examine the molecular geometry, or form, of the molecule. Polarity is caused by an uneven distribution of valence electrons in the atoms. SF2 molecules are not perfectly symmetrical, hence there is an uneven sharing area in the molecule.
[Solved] SF2 does this compound contain polar covalent bond or nonpolar
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape.

Sf2 Lewis Structure Hybridization Sf2 molecular geometry and lewis
Are you curious about whether SF2 is polar or nonpolar? Look no further! In this article, we will explore the molecular structure of SF2 and delve into the factors that determine its polarity. By understanding the chemical behavior and interactions of SF2 with other molecules, we can determine whether it possesses a dipole moment. Using… Read More »Is Sf2 Polar Or Nonpolar?
Is SF2 Polar or Nonpolar? YouTube
Question: Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. There are 2 steps to solve this one.
Sf2 polar or nonpolar jujapress
Now in the next step we have to check whether these Se-F bonds are polar or nonpolar. And we also have to check the molecular geometry of SeF2. Step #2: Check whether individual bonds are polar or nonpolar. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the.

Is SF2 Polar or Nonpolar? Techiescientist
Have you ever wondered whether SF2 is a polar or nonpolar molecule? If so, you're not alone. This question has puzzled many chemistry students and professionals alike. In this article, we'll explore the properties of SF2 and determine once and for all whether it's polar or nonpolar. What is SF2?
Sf2 polar or nonpolar limfath
Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.
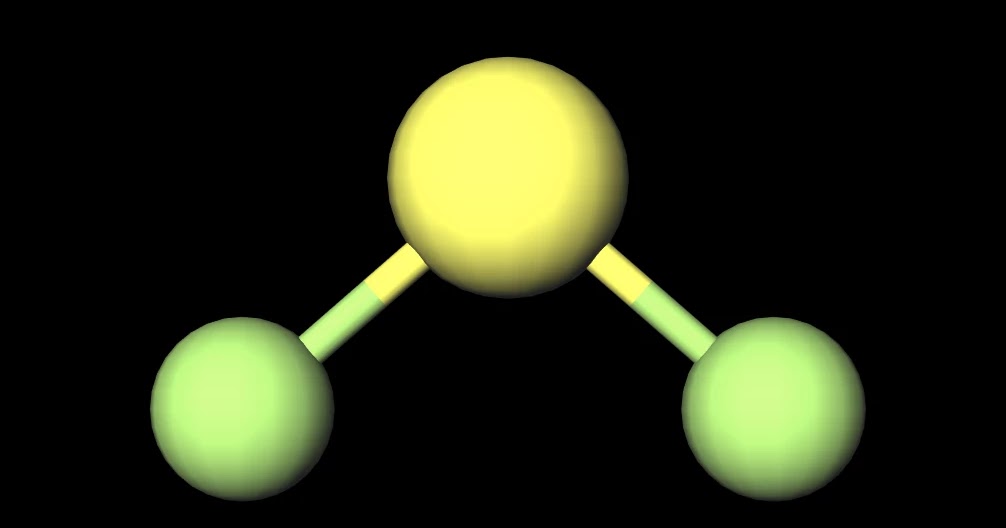
MakeTheBrainHappy Is SF2 Polar or Nonpolar?
Step by Step Construction of Lewis Structure SF2 Molecular Geometry SF2 Lewis Structure- Key Points SF2 Hybridization Is SF2 Polar or Nonpolar? Related Links Summary Frequently Asked Questions (FAQs) More Links Step by Step Construction of Lewis Structure Following are the steps to construct the Lewis Structure.